The history of AvDesk®
AvDesk® is a class I medical device for the development of neurovisual rehabilitation therapies.
Born in 2010 from the multi-year work conducted by Linari in collaboration with IRCCS Stella Maris Foundation Pisa, world leader in child neuropsychiatry, AvDesk is a device protected by an international patent and uses state-of-the-art technologies to work in telemedicine.
AvDesk allows doctors and therapists to develop a cognitive training path customizable day-to-day on the needs of the assisted person, ensuring appreciable improvements already after two weeks of treatment.
10 years ago, Italian researchers and IRCCS researchers had evidence that some children with congenital visual field deficits could not carry out activities in the blind spot.
“Through Nuclear magnetic resonance, neurologists had found that their areas of vision corresponding to the blind angle were active: the solution must therefore be of neurological origin and deriving from synaptic plasticity, which is maximum in childhood and which naturally managed to compensate part of the deficit”, explains Dr. Caterina Stimola, CEO of Linari Medical.
Remote use was necessary for these little patients to remain in the facility for the bare essentials and then continue their rehabilitation at home. The device therefore had to be simple in order to be used even without the presence of a doctor.
In the case of uncooperative patients, AvDesk works without a button, thanks to the Gaze and Head Tracking system which verifies in real time the vision of the stimulus and the contribution of the patient’s head in the search for the stimulus.
AvDesk uses cutting-edge technologies to work in telemedicine and allows doctors and therapists to develop a cognitive training program personalized daily on the needs of the assisted person, guaranteeing appreciable improvements already after two weeks of treatment.
AvDesk is featured in the August 13, 2018 episode of SuperQuark (Rai 1) “How the Brain Sees” presented by Piero Angela. The concepts of brain plasticity for the rehabilitation of children are explained
International patent
Europa

Svizzera

USA

Giappone

AvDesk: to whom is it aimed?
AvDesk can be used on collaborative and non-compliant patients, adults and children affected by neurovisual deficits such as: hemianopsia, neglect, consciousness deficits, Alzheimer, cognitive and attention deficits.
It is a unique device of its kind to carry out the clinical evaluation and treatment of these alterations, an international reference point for neurovisual deficits.
Cases in the world
4+ Million
Hemianopia and Quadrantanopia
Cases in Italy
7 Million
Hearing impairments
The prevalence of auditory problems in Italy is estimated at 12.1% of the population, about 7 million Italians with hypoacusis.1.5 Million
Visual deficit
By 2030 an increase of blind population about 25% due to the ageing of the population.276.000
Attention or learning deficits
Treatment may also be aimed at improving the cognitive capacity of children with deficits affected by DSAs.600.000
Alzheimer
The incidence of this disease is destined to increase because of the rise in life expectancy.Principles of operation
AvDesk is a multisensory telerehabilitation device that exploits brain plasticity to stimulate intact subcortical structures. Through the stimulation panel a series of visual and sound signals are delivered, programmed according to sequences and temporal frequencies determined by a personalized therapy.
Fields of application

Tele-neurovisual rehabilitation
Patient undertakes a cognitive training process which, through audiovisual stimuli, allows to compensate for neurovisual deficits such as: Hemianopia, Neglect, Attention deficit disorder, Alzheimer and cognitive deficits.

Skill test
AvDesk continuously monitors the results achieved by patients customizing their
treatment of individual rehabilitation or cognitive development daily. To do that, AvDesk
takes advantages of the most innovatives computer vision systems.

Cognitive development
The AvDesk therapy could lead to a cognitive development which can significantly
improve the peripheral vision response time. This can be useful for those looking for better
sport performances or those in needs of special visual skills as athletes, rescue teams,
airline pilots, drivers or army forces.
Tele-neurovisual rehabilitation
Patient undertakes a cognitive training process which, through audiovisual stimuli, allows to compensate for neurovisual deficits such as: Hemianopia, Neglect, Attention deficit disorder, Alzheimer and cognitive deficits.
Skill test
AvDesk continuously monitors the results achieved by patients customizing their treatment of individual rehabilitation or cognitive development daily. To do that, AvDesk takes advantages of the most innovatives computer vision systems.
Cognitive development
The AvDesk therapy could lead to a cognitive development which can significantly improve the peripheral vision response time. This can be useful for those looking for better sport performances or those in needs of special visual skills as athletes, rescue teams, airline pilots, drivers or army forces.
AvDesk: how does it work?
This treatment is based on the multi-sensorial stimuli of untouched subcortical structures. Through a stimulation panel the patient reacts to a variety of audio-visual signals. How often and in which sequence these signals are given depends on the patient progresses and is adjusted in time. A wireless button with a feedback interface allows the patient to answer these stimuli.
The personalized treatment is daily modified and re-adjusted by a qualified doctor certified by Linari, connected on remote. Years of researches have allowed Linali Medical to create its own analysis software which continuously sends treatment result feedback after every dosing.
The device measures the time frame in millisecond between the lighting of the stimulus (anticipated by an acoustic signal) and the pushing of the wireless button by the patient.
Therapy benefits are also highlighted in the reduction of patient response times for the peripheral areas of his visual field too.
Effectiveness of the treatment is ensured by AvDesk use in a quiet and half light location where audiovisual stimuli are clearer.
Download brochureAn IR camera constantly monitors the patient's attention level
Lightweight and easy to install thanks to its rollable structure
Constant control through telerehabilitation
Doubling of visual abilities:
1h a day (also non-continuous) for 20 days
Treatment of Hemianopsia
Subject aged 15: left hemianopia. Average response time and visual sensing capabilities.
Teal-time graphics processed by the Linari Medical Cloud platform.
Before therapy
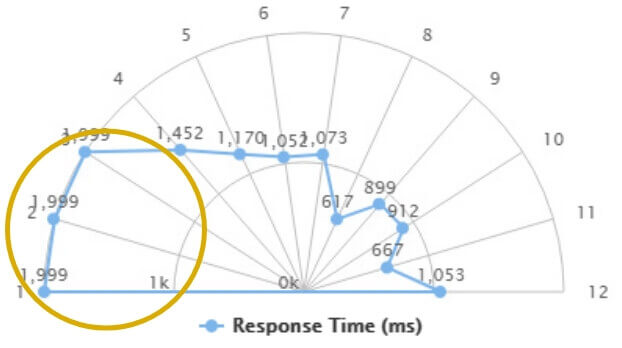
The subject cannot see the light stimuli on the first three segments on the left.
A time of 1,999 ms identifies an absence of response from the subject.
After therapy
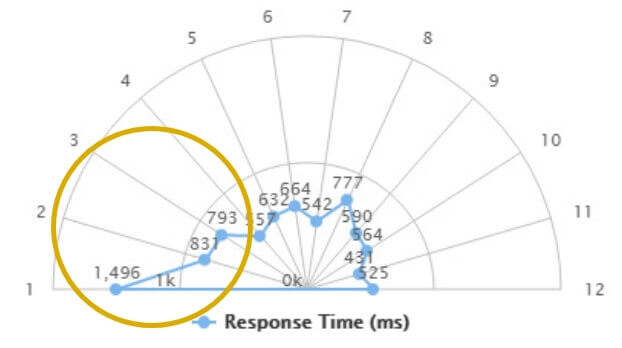
There is a significant improvement in response times to stimuli coming from the patient’s blind side. The whole visual field of the subject also benefited from the treatment.
Tracking head and eye movements
AvDesk: measure of residual cognitive abilities
Both the gaze and the head are aligned with the stimulus, both gaps are zero because the patient has performed the therapy correctly.
Tracking of stimuli with the head and with the gaze
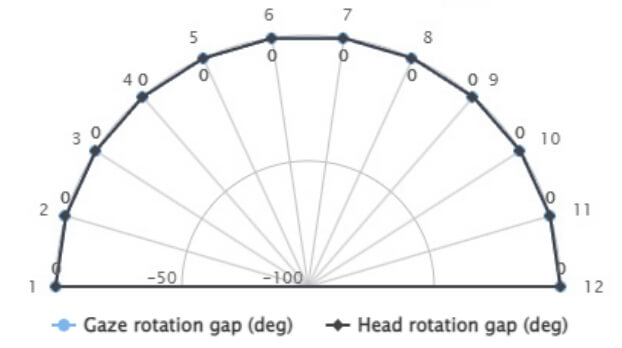
The subject cannot see the light stimuli on the first three segments on the left.
Chasing the stimuli with the gaze and the head still in the centre
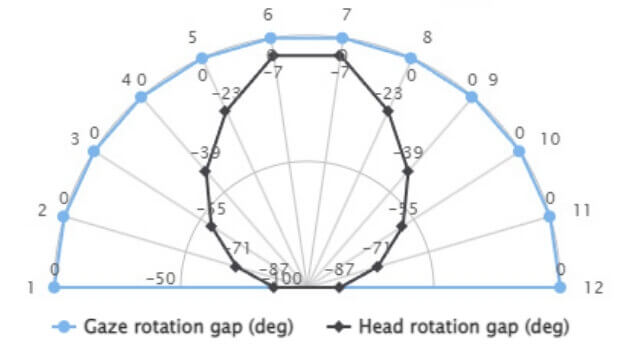
The gaze is always aligned with the stimulus (zero gap), while the head, remaining fixed in the center, has a decreasing alignment gap towards the ends of the visual field. The negative sign is given by the less rotation of the head with respect to the position of the stimulus.
Do you want to learn more about results and studies?
Download brochureTele-neurovisual Rehabilitation
Compact, light and easy to carry thanks to its travel case, AvDesk has been designed to fully exploit all advantages offered by telemedicine and make it possible to use it directly at the patient's home.
The device guarantees therapy adherence without the physical presence of the tutor, thanks to an integrated artificial visual system based on sophisticated algorithms which can recognise the patient’s attention level, keeping his focus on the ideal treatment conditions. Being the system based on brain plasticity, naturally present in each individual, no medicine ingestion is necessary.
Thanks to a secure access to the Linari Medical cloud, the doctor verifies his patients’ progresses in real time to support them in a framework of neuro-functional and, possibly, psycho-motor rehabilitation.
The data processing system on which the device is based makes it possible to exploit the potential of teleconsultation. Specialists and experts in the health sector can consult, evaluate and modify the therapies, adapting them to the progress of the treatment.
AvDesk at home

AvDesk in clinic
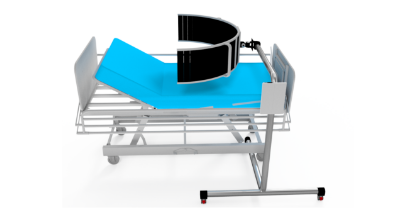
AvDesk in medical office

Practical and user-friendly
AvDesk consists of a stimulation panel that can be rolled up on itself, light and easily foldable using only one hand. In its entirety, the device fits comfortably on a desk.
The Linari Cloud software continually updates the AvDesk device with new therapy algorithms through the internet connection.
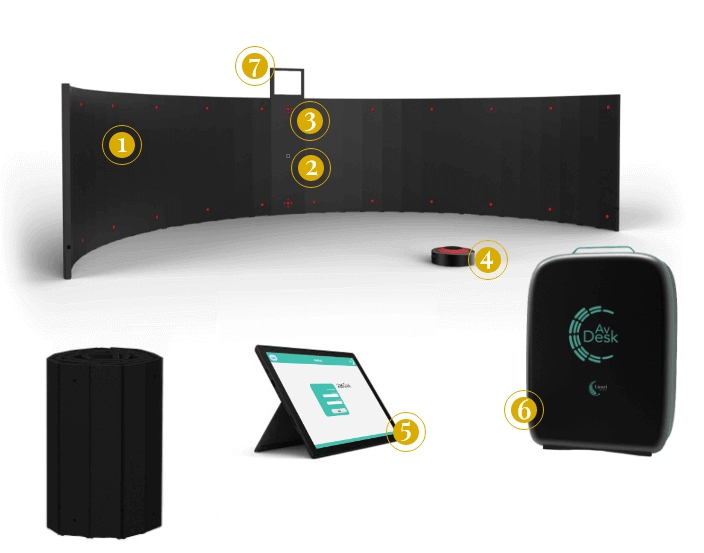
1 - Stimulation panel
A curved 180 degrees screen stimulation panel that emits sound and visual stimuli. Sound and light can be spatially coincident or out of synch.
2 - Camera
An high-resolution with night vision camera, integrated with the main panel on the curved monitor and connected with the Data Collection Unit, to control the bright arrows group during the whole patient monitoring process.
3 - Indicator arrows
Four bright arrows group which lead patient gaze to the center part of panel control after every stimulation, to guarantee the treatment effectiveness.
4 - Wireless button
Signal Interface (wireless button) pushed by the patient upon stimulus reception.
5 - Microsoft Surface
The Linari Medical Cloud platform has an intuitive interface for managing therapies and monitoring results through reports and graphs.
6 - Dedicated mini trolley
For easy transport AvDesk can be rolled up and stored in a mini trolley with standard sizes, even for air travel.
7 - Calibration mirror
To calibrate the ambient light and make the visual stimuli sent by AvDesk more evident.

Linari Medical Cloud
Full access to your personal space on the Linari Platform Medical Cloud, makes it possible to:
Create a profile of the patient (with customizable password) and add their medical record:
- set a standard parameter of the axis of rotation of head and eyes, tailored to the needs of
said patient; - activate or deactivate Face Tracking based on the patient’s attention level.
Start the first session to analyze the starting level of the assisted subject.
Customize the therapy, adapting the position and volume of the auditory stimulus, its position and the visual stimulus frequency, as well as the colour spectrum (over 16 million) and brightness of each LED panel. Checking for coincidence or spatial asynchrony between auditory and visual stimuli, false positives, giving priority to the visual stimulus compared to the auditory stimulus or vice versa.
Plan a therapy schedule for 2-3 days.
Read and download summary graphs of the individual sessions and use them when assessing the starting level of the assisted subject, to adapt the therapy in the basis for the evolution of the patient’s clinical status.
Download tabular response time reports, including detailed information:
- number of the panel segments associated with the reproduction of visual and auditory stimuli;
- frequency of repetition of stimuli on each segment;
- success rate the identification of stimuli;
- response times of the subject for each stimulus)
Create new customized protocols.

Scientific Publications
More than 15 years of research were dedicated to the development of compensatory rehabilitative treatment for Hemianopia.
The validated results were published in relevant medical journals.